Latest Press Releases

Advarra will integrate Ethical’s innovative and flexible solutions into its next-generation SOAR platform for the effective, compliant, and safe management of Data Management Committee (DMC) and Endpoint Adjudication Committee (EAC) services.
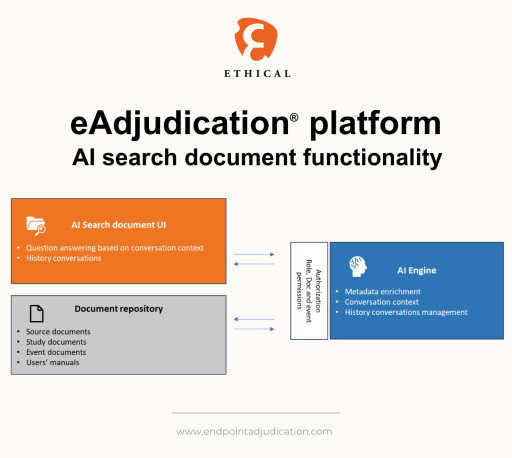
By leveraging advanced natural language processing and machine learning capabilities, Ethical’s new AI-powered conversational documents search tool reduces the time and effort required to find critical information and enhances the overall efficiency and quality of the endpoint adjudication process.
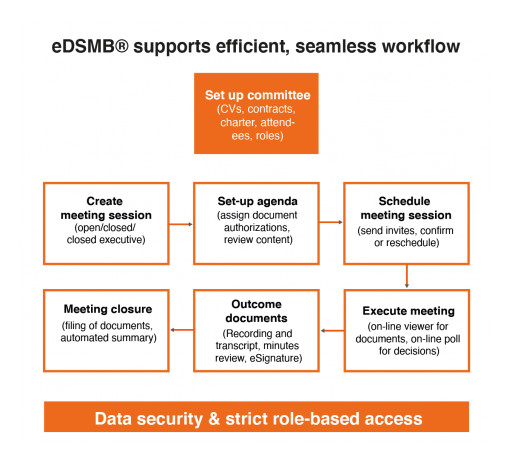
The new Ethical eDSMB® software provides sponsors, CROs and AROs with a comprehensive solution for easier and more efficient DSMB, DMC and Steering Committee management. With eAdjudication® for clinical adjudication, eDeviation® for protocol deviations, and now eDSMB® for DSMBs, DMCs and Steering Committees, Ethical's customers have access to a full range of easy-to-use software platforms for managing clinical committees in a more efficient way.
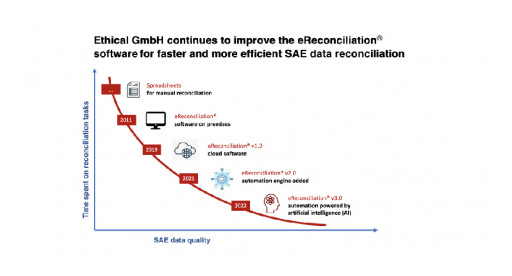
Ethical GmbH releases a new version of eReconciliation® that uses artificial intelligence (AI) to improve the performance of the software automation engine. This third version of eReconciliation® continues to optimize time spent on SAE data reconciliation and reduce the risk of errors and omissions, particularly when high volumes of data or heterogeneous environments are involved.

The eAdjudication® electronic endpoint adjudication platform is upgraded with an innovative Zoom connector that allows it to streamline the management of endpoint adjudication meetings, and safely and conveniently file meeting recordings and transcriptions within the platform.
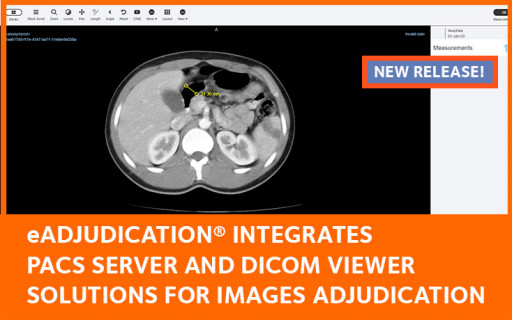
The new DICOM / PACS extension to the eAdjudication® electronic endpoint adjudication software brings on board the Orthanc server and a fully-equipped DICOM image viewer. This comprehensive solution facilitates the acquisition, storage, transmission and display of DICOM images for Expert Committees.

Ethical GmbH announces a new software solution for the assessment and management of protocol deviations in clinical research. Focusing on protocol deviations, Ethical's new eDeviation® software completes the company's existing offer of GxP-compliant, secure, and validated SaaS solutions supporting simple, effective, and compliant clinical trials management.
Blockchain Technology Improves Quality and Data Integrity of Ethical's eAdjudication® Software for Clinical Endpoint Adjudication
Stanford Center for Clinical Research Adopts Ethical eAdjudication® Cloud-Based Software Service for Clinical Endpoint Adjudication
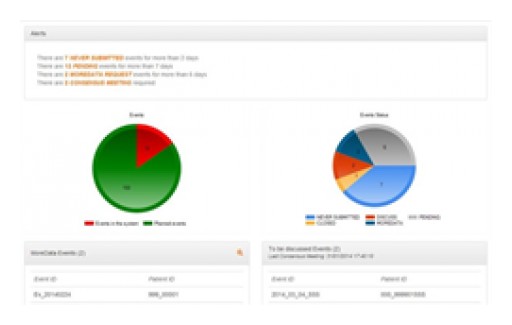
Uppsala Clinical Research Center (UCR), a top Academic Research Organization based in Sweden, shares its experience in using the Ethical eAdjudication® cloud-based platform to manage the central assessment of Clinical Trial Endpoints by an independent CEA (Clinical Event Adjudication) Committee.
